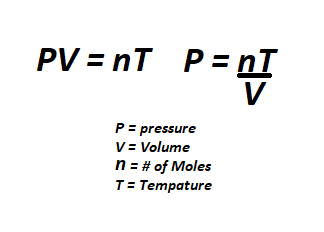User:Nico64/sandbox
Atmospherics (simply shortened to atmos) is one of the most complex fields of the entire station, and the job of the atmospheric technicians.
Basic atmospherics
All gases can flow through the various pipes found in the game. Gas will always attempt to flow from higher pressure to lower pressure. If a gas is not in a pipe, canister, or tank, it will be in the atmosphere and will interact with other objects.
Gas will always try to even out the pressure. For example, if a empty canister is connected to a pipe pressurized at 4500kpa, the canister will also only be pressurized to 4500kpa. If a canister pressurized at 9000kpa is connected to the same pipe, gas will flow out of the canister until a even pressure is acquired.
If pressurized pipes get unwrenched they will dump all of their contents into the surrounding atmosphere and will, depending on the pressure level, violently blow the wrench user away. You will know if you are unwrenching a pressurized pipe if you get the message stating "A gush of air blows in your face... Maybe you should reconsider?" Alternatively, you should always use your gas analyzer on every pipe before unwrenching.
All pipes can be unwrenched to disconnect them from others. By using a welder on a unwrenched pipe segment you can deconstruct it into steel.
A broken or unconnected segment of pipe WILL NOT allow gas to pass through. Do not worry about all your gas escaping out of a broken or unconnected pipe segment.
Most pumps, mixers, and filters do not require power to function. Only air vents and scrubbers require power. You can shift-click on a segment to examine it to see if it is powered.
Pressure and moles
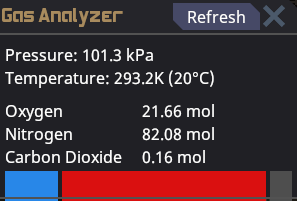
All gas can be quantified by its pressure, mole amount, and temperature. These three variables are closely related and directly affect one another. If you add more gas(# of moles) to a given area, the pressure will increase. Take the same amount of moles and lower the volume by using a smaller room and the pressure will be even larger. If you then heat up the gas, the pressure will be even larger still. The opposite is also true, less gas means lower pressure. cooling down gas will also lower the pressure. Volume, or size of the room, also plays a role in pressure. A larger area will require more gas while a smaller area will require less gas to reach the same pressure. using this knowledge we can see why space has a low pressure because the area and temperature are so low.
Moles are a way of measuring how much gas is present in a given area. A higher pressure does not always mean more of a gas in the given area. If you want to physically fit a larger amount of a gas in a specific area, you will need to cool the gas down to lower the temperature, thus lowering the pressure allowing more moles to fit inside the given area.
If you open a canister will 100 moles of air into a large hallway, you will hardly notice a difference in pressure. However, if you open the same tank in a small room the pressure difference will be greater.
The standard livable air requirements are about 20 moles of oxygen and 80 moles of nitrogen at a pressure of 101kpa and temperature of 20° Celsius. If you have less than 20 moles of oxygen present, your character will begin to gasp and take oxygen deprivation damage. If the pressure is any lower or higher than 101kpa, your character will begin to take brute damage in relation to the depressurization or overpressurization levels. If the temperature is much lower or higher than 20°C, you risk your character taking burn damage from the extreme cold or heat.
Generally speaking:
- More gas(# of moles) = more pressure.
- Less gas(# of moles) = less pressure.
- Hot gas = more pressure.
- Cold gas = less pressure.
- Large hallway = more moles/higher temperatures needed to notice pressure change.
- Small hallway = less moles/higher temperatures needed to notice pressure change.
Atmospheric Devices
Pipes
Allows gas to flow freely. Comes in four shapes: Straight, Elbow, 3-way-juntion, 4-way-junction.
Pumps
Pumps gas to the other side based on the set pressure. Blocks gas from passing through if toggled off. Comes in two varities:
Standard gas pump. Pumps gas along based on internal and external pressure. Has a maximum throughput of 4.5 Mpa. Mostly used to act as a valve to allow/disallow gas to flow from one pipe to another. Works very well in high pressure pipes but looses effectiveness when the pressure is lower.
For low pressure pipes a volumetric pump is generally a better option.
Volumetric pump. Pumps gas along based of internal and external mole amount. Has a maximum throughput of 200 L/s. Acts exactly like a gas pump but works off of mole amount instead of pressure. can move about twice as much gas compared to a regular gas pump if conditions are right.
For extremely high pressure pipes a regular gas pump is generally a better option.
In certain cases volumetric pumps are also better if you are using huge quantities of gas.
Manual Valves
Acts like a switch that either allows gas to flow through, or prevents it from flowing through. Can be toggled on and off.
| Image | Name | Description |
|---|---|---|
| Manual valve open | A green light is open. | |
 |
Manual valve closed | A red light is closed. |
Gas Mixer
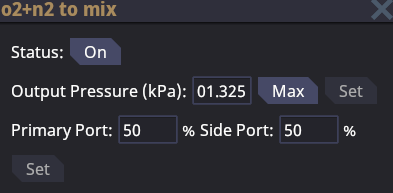
A gas mixer is a fancy version of the gas pump. It allows you to combine the gas flow of two different pipes and mix them into a single pipe. The gas mixer will combine the contents of two different pipes and will give you the option of setting the percentage of throughput you want on each of the input pipes. The primary port will be parallel with the output while the side port will be perpendicular.
Gas mixers are essential for distro as the station's atmosphere is 20% oxygen and 80% nitrogen. Gas mixers are also used for the combination of two different gases to create a new gas such as a common burn-mix.
If the gas mixer is toggled off then it will not allow any gas to flow through.
If only one port is connected the gas mixer will not function. Both input ports must be connected and have gas flowing through them in order for the mixer to have a output.
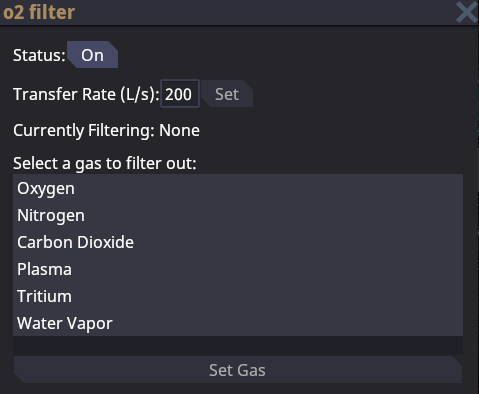
Gas Filters
Gas filters are another special type of gas pump. Gas filters are primarily used to extract a specific individual type gas from a pipe. They function similarly to the gas mixer, except they have one inlet port and two output ports. When gas flows through a mixer the selected gas to be filtered out will exit the perpendicular outlet port while all other gases will continue to flow down the parallel outlet port. If you wish to filter out more than one gas you will need to set up gas filters in series for each specific gas.
Gas filters are used to extract each individual gas from the waste loop. This ensures that only one type of gas is present in each specific gas holding tank. Gas filters are essential when you are trying to target and isolate a specific gas.
Gas filters do not require both outputs to be connected to function if there is no gas selected to filter or the filtered gas is not present. In this case the filter will act as a simple straight pipe segment. However, The filter will not allow gas to flow if the selected gas is present and there is no pipe connected to the perpendicular filter port.
Vents
Gas vents come in three varieties: Standard, Dual-port, and Passive.
All gas vents are solely used to move gas from pipes into the surrounding atmosphere. Gas vents are commonly used in conjunction with the distro loop to distribute breathable air to the station. Gas vents and the distro system are responsible for replenishing the atmosphere on the station.
A standard air vent will only allow a maximum pressure of 101.7 kPa to flow. If the external pressure is higher than the limit, no gas will flow out of the vent. Requires power to function.
A dual-port air vent is exactly like a standard air vent but it has two inlet connection ports.
A passive air vent does not require power and will allow any pressure to flow in both directions depending on the internal and external pressures. If the internal, or pipe pressure, is higher than the external, or outside atmosphere, Gas will flow out. The opposite is true if the external pressure is higher than the internal pressure. Passive air vents are mostly used in the mix tank and Supermatter chamber where very high pressures are needed.
Air Scrubber
Air scrubbers slowly suck gas out of the surrounding atmosphere and can be found spread around the station. Mostly used in the waste loop, air scrubbers will syphon gas out of the atmosphere and shunt it along the waste loop where the gas will then be recycled and filtered into the gas holding chambers.
Air scrubbers must be powered and connected to an outlet port in order to function. A properly functioning air scrubber will be glowing blue.
Connecter Port
Connecter ports are solely used to transfer gas from a pipe into a canister and vice versa. Wrench a canister on top of a connecter port to connect them. A connected canister will allow gas to flow until the pressure evens out.
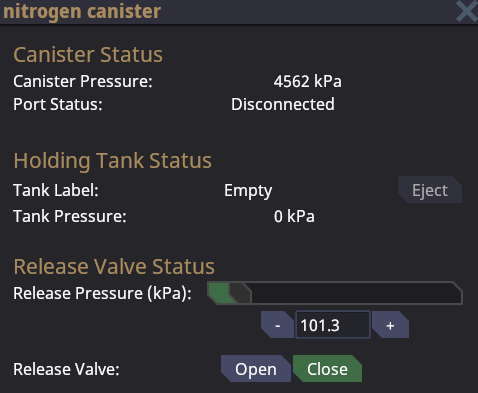
Canisters
Canisters are used to hold and transport gas. Drag a canister over to a connecter port and use a wrench to connect it. If the connecter port's internal pressure is higher than the canister pressure, gas will flow into the canister until the pressure evens out.
If the canister has a higher pressure gas will flow out until the pressure difference is even. Canisters can release gas in different ways depending on the situation. Gas will only flow out if the release valve is toggled open. If the release valve pressure is lower than the external pressure, no gas will flow out.
- If the canister is wrenched to a connector port, gas will flow into the connector port.
- If a oxygen tank is inserted into a canister, gas will flow into the oxygen tank.
- If the canister is not connected to anything, gas will flow out into the surrounding atmosphere.
Gas Miner
Gas miners create new gas from nothing and are currently used to make sure the station has an infinite amount of a specific gas. Gas Miners come in different types and create different types of gas. A oxygen miner will create oxygen gas while a plasma miner will create plasma gas. Gas Miners can be found in Atmospherics inside each gas holding chamber.
Gases
Nitrogen
Nitrogen (N2) gas is colorless and undetectable to the eye. Nitrogen gas is completely harmless on its own and can safely be breathed in as long as there is adequatea oxygen present. Nitrogen gas is mixed with oxygen gas to create air. About 79% nitrogen to 21% oxygen is the standard air mix.
Nitrogen is very good at putting out fires. A full blown plasma and oxygen fire can be put out in seconds if a small amount of nitrogen gas is pumped in.
Oxygen
Oxygen gas (O2) is colorless and undetectable to the eye. Oxygen gas is completely harmless on its own and can be safely breathed in. Pure oxygen can also be breathed in without any negative effects on your character. Oxygen gas is mixed with Nitrogen gas to create air. About 79% nitrogen to 21% oxygen is the standard air mix. As the oxygen levels in the air lower your character will begin to gasp. This usually happens around 18-19 moles of oxygen. Anything lower than 18 moles of oxygen in the air and you will begin to take oxygen deprivation damage. While alive, your character will slowly convert oxygen gas in the atmosphere into carbon dioxide. If a character spends a lot of time in a room make sure the oxygen levels are being replenished either by a air vent or by opening adjacent airlocks to nearby hallways to balance out the air levels.
Unlike in real life, Oxygen gas is not flammable on its own and will not burn unless plasma is mixed in with it. A standard burn mix is 90% oxygen and 10% plasma. A higher oxygen to plasma ratio will result in a hotter burning fire. Fires require oxygen to burn, remove all the oxygen from a fire and it will burn out.
Carbon Dioxide
Carbon dioxide (CO2) is colorless and undetectable to the eye. Carbon dioxide is completely harmless on its own and can safely be breathed in as long as there is adequate oxygen levels present. Carbon dioxide is often found mixed in the air around the station. Your character will slowly convert oxygen into carbon dioxide while alive.
Water Vapor
Water vapor (vaporized H2O) can be clearly seen as steam. the intensity of visible steam depends on the concentration of water vapor present in the air. Water vapor is completely harmless and can safely be breathed in as long as there is adequate oxygen levels present.
Plasma
Plasma gas glows a pinkish purple and is visible in the air even at low amounts. Breathing in plasma gas is harmful to your character and will give you poison toxin damage depending on the concentration of plasma in the air.
Plasma gas is extremely flammable but on its own will not burn. A room full of pure plasma will not burn if heat is brought into contact. Plasma requires an oxygenated room for combustion. Plasmafires burn oxygen and plasma to produce fire, heat, and waste gases. A plasma fire will quickly deplete the room of oxygen and will burn itself out if no more oxygen is supplied to feed the fire.
Tritium
Tritium is a product of the combustion of plasma and high concentrations of oxygen. When exposed to more oxygen, it will burn and turn into water vapor. When created, it will likely be extremely hot and will burn anyone exposed to it to a crisp.
A popular ratio to create it is 97% O2 : 3% Plasma, or 85% O2 : 15% Plasma.